Hospital – Wound Treatment: Algoderm Trionic® (Japan)
Algoderm Trionic® (Japan)
- Bioactive Medical Device for Wound Therapy and Haemostasis
- Suitable for deep wounds that reach the bone
Embedded in a hydroactive biopolymer matrix, Manganese, Zinc and Calcium ions accelerate biological wound healing processes in all phases.
Those important enzyme co-factors are released in a controlled manner through ion exchange and migrate into cells involved in tissue regeneration.
Algoderm Trionic® (Japan) is effective to stimulate the blood clotting and wound healing.
Indications
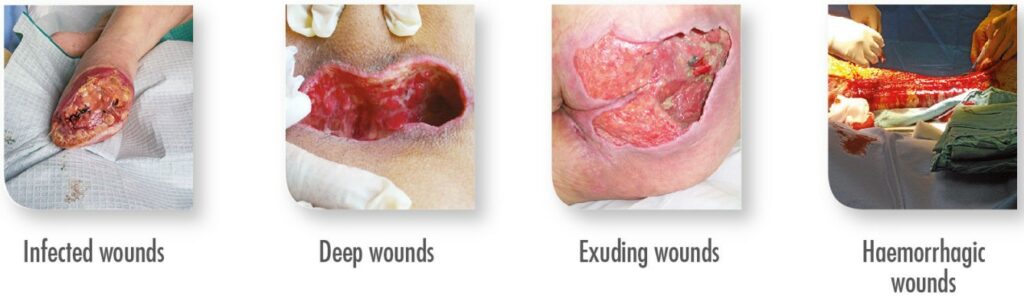
Chronic wounds (e.g., diabetic foot, pressure sores, leg ulcers), traumatically caused wounds (e.g., intra- and postoperative bleeding, burns, surgical wounds), deep wounds, and wound cavities.
Bioactive Medical Device for Wound Therapy and Hemostasis
- Bio-active matrix enriched with 3 ions: Manganese, Calcium and Zinc
- CE marked in class III
| INDICATIONS | MECHANISM OF ACTION |
| Healing Moderate to heavily exuding wounds
|
Upon contact with the wound, ALGODERM TRIONIC absorbs exudate and traps necrotic debris and bacteria 1,2,3 in its interfibrilar matrix, thus promoting wound cleansing and granulation 4,5,9. At the same time, ALGODERM TRIONIC frees its zinc, calcium and manganese ions into the wound bed. The 3 ions stimulate key cells of the healing process and fasten tissue regeneration 4,5,6,7,8,9. Finally, upon contact with wound fluids ALGODERM TRIONIC gelifies and maintains a moist environment required for proper wound healing. ALGODERM TRIONIC is designed to minimize pain 2,3,9 and facilitate an easy removal, without damaging the newly formed tissue. |
| Haemostasis ALGODERM TRIONIC is efficient in patients with bleeding disorders whether congenital or acquired
|
Upon ontact with the wound, ALGODERM TRIONIC absorbs sodium ions from the blood and frees its zinc, calcium and manganese ions into the wound bed. This process is called IONIC EXCHANGE. The 3 ions released into the wound stimulate platelets activation and aggregation, and contribute to the chain reaction of the coagulation cascade. ALGODERM TRIONIC significantly accelerates haemostasis 7,8,9. |
ALGODERM TRIONIC can be used as interface with NPWT devices (follow the instructions of the device manufacturer).
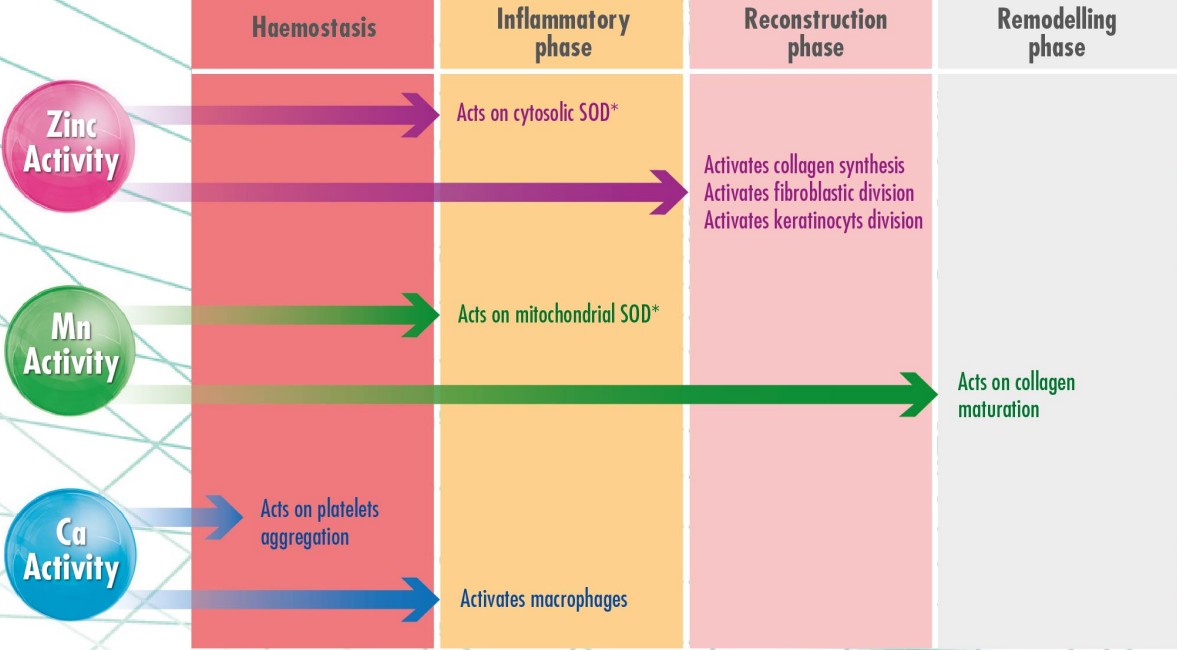
*S0D (Superoxide dismutase) protects the cells against free radicals
ALGODERM TRIONIC enhances fibroblastic division by 50%, collagen type 1 production by 300% and collagen type Ill production by 400%⁶.
References
- Kinetic binding of bacteria on two types of dressings: Algosteril® and Gouze, l st European workshop surgery-engineering: synergy in biomate-rial applications, Montpellier 19-20 May, 1994.
- Algosteril® dressing vs. Dextranomere in the treatment of Decubitus Ulcers at 20 French Centres. 8th Annual Symposium on the Treatment of the Wound, San Diego, USA, May 1995.
- Algosteril® vs. Povidone lodine dressing in the treatment of infected wounds. IXth European Congress of lnfectious Surgery, Paris, June 1996
- ZIEGLER U.E. et al. The treatment of chronic wounds with a new calcium-zinc-manganese alginate dressing. Fortschritte der Medizin 121. Jg. – Originalien Nr. l/2003, S. 19-26.
- TARIN SAEZ J.J. et al. Effectiveness of bioactive dressing with ionic charge in the reduction of healing time in chronic wounds. Metas de Enferm 9(1): 58-64, February 2006.
- CASTELLARNAU C. et al. The activating function of bioactive dressing with ionic charge on human fibroblasts. Metas de Enferm 8(7): 50-54, September 2005.
- SERVANT J.M. et al. Calcium polyuronate dressing supplemented with zinc and manganese (TRIONIC®) in necrotizing dermohypodermitis of the extremi-ties: a randomized multicenter study. Annois of Burns and Fire Disasters, Vol. XXIII, n.2, June 201 O.
- Algosteril®, calcium alginate, vs. Vaseline Gouze in the Treatment of Excised Lesions of Verneuil’s Disease. 4th Australian Wound Management Association Conference, Morch 2002.
- PANNIER et al., Efficacy and tolerance of Algosteril® vs. Jelonet® in the treatment of scalp graft donor sites in children. Annales de Chirurgie Plastique Esthétique 47 (2002) 1-6.
ALGODERM TRIONIC ® is developed and manufactured in France by Les Laboratoires BROTHIER, 41 Rue de Neuilly – 92200 Nanterre, France.
ALGODERM TRIONIC ® is a trademark of Les Laboratoires BROTHIER.
For more information about ALGODERM TRIONIC ®, please call +33 (0) 1 56 38 30 00 or email at info@brothier.com.